Measuring the impact of patient engagement: How pharma can lead the way
“You can’t improve what you don’t measure,” as the famous business motto goes. Measuring the impact of patient engagement has been challenging health stakeholders for years. Evidence of impact is still often criticized as being weak and anecdotal.

The significant increase in patient engagement in recent years – among all health stakeholders – suggests that there is clear perceived value, no matter how intangible. The perspectives of patients and carers are now widely and routinely incorporated into the design of healthcare trials, programs, and policies globally.
On a macro scale, healthcare systems are increasingly organized around people’s needs rather than around what health services can offer, and there are tools in place to collect and share what matters to patients. The Patient-Reported Indicator Surveys (PaRIS) initiative from the OECD, for example, aims to provide systematic, internationally standardized information on what matters most to patients, using indicators that enable comparisons across countries.
The pharmaceutical industry strives to consistently integrate the patient perspective throughout the research and development (R&D) process. Patients are regularly consulted around the design of clinical trials, and the number of studies incorporating patient reported outcomes (PROs) has rocketed, from only 25 between 1992 to 2002, to 7,464 studies from 2002 to 2022. For rare diseases such as spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD), patient expert input has been invaluable in identifying outcomes that are relevant and meaningful to people living with these conditions.
These patient data and perspectives are increasingly being recognized by regulatory bodies too. The FDA has developed a patient-focused drug development guidance series to enhance the incorporation of the patient voice in regulatory decision making, and the EMA is seeking to do something similar. Patient experience data was reported as relevant for 39 out of the 48 (81.3%) medicines approved by the FDA in 2019, while 100 out of 128 (78.1%) oncology indications approved by the EMA between 2017 and 2020 included PROs in the confirmatory clinical trials.
In terms of health technology assessment (HTA), most agencies are incorporating patient perspectives more systematically in their processes. For example, the National Institute for Health and Care Excellence (NICE) in England published its Real-World Evidence Framework in 2022, establishing its approach for utilizing real-world data to resolve gaps in evidence, including patient experience data.
All of this seems very positive. But the question remains – what impact is any of this actually having?
Measuring impact remains elusive
In each example of patient engagement mentioned above, the value seems clear, intuitively at least. Having patient and carer voices around the table brings authenticity and weight to decision-making about products or services that will directly impact their lives. Whether it’s a clinical trial, a product launch, legislation or strategy, early integration of the patient perspective can help to overcome roadblocks and weaknesses that were previously exposed during implementation.
But how can we demonstrate this? What are the tangible metrics? How can we measure the impact of patient engagement?
Despite some progress in this area, gaps remain, and more robust evidence is still required. One of the main long-standing challenges with assessing the impact of patient involvement is the lack of agreement as to what precisely constitutes a return on engagement from patient activities and how to evaluate the impact.
Now, a key drive in this area is to demonstrate the tangible impact of such engagement based on what matters to patients themselves. Metrics such as the number of patient engagements, for example, may be meaningless if little or no value to patients is delivered.
Measuring what matters
So, how can we measure what really matters to patients?
First of all, the objective and desired impact of engagement for each stakeholder needs to be co-defined with patients to ensure they are truly meaningful. It is the responsibility of all stakeholders to make this happen as a foundation for future engagement.
There are some existing tools to help to facilitate this process. For example, PARADIGM, funded by the Innovative Medicines Initiative (IMI), provides a toolbox to help measure the value of collaborations between patients / patient organizations and a range of stakeholders. This provides a valuable starting point, but it is important that measurements are tailored to the needs of the specific disease area.
From the foundation of the patient-centric objective, KPIs (key performance indicators) and metrics can be developed to inform the journey to achieving the objective. A KPI is a quantifiable measure of performance over time for a specific objective, whereas a metric is an indicator related to activities along the journey. It is typically easier to measure than a KPI and useful as a proxy to understand progress.
In Figure 1, we have provided examples of patient-centric objectives for each stakeholder group, along with metrics to help measure the progress towards KPIs for each one. For policymakers for example, the ultimate objective may be to create a patient-centered health framework to deliver meaningful healthcare. The KPI to demonstrate this has been achieved is the impact of patient insights on healthcare priorities and service provision. The metric to help gauge progress towards that KPI (which can be measured at multiple time points) is the number of decisions integrating patient input.
Figure 1: Patient-centric objectives by stakeholder
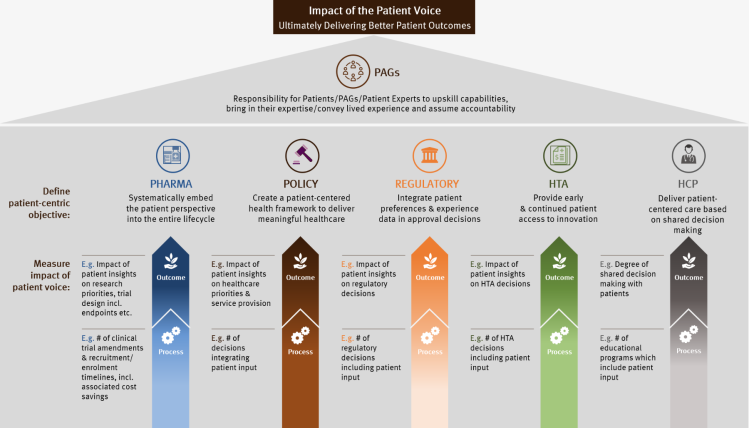
Most importantly, the objectives, KPIs and metrics must be co-created with patient communities. And once they have been agreed, patient communities should then be systematically involved throughout the process to support and play their part in helping to achieve them.
With patient communities becoming increasingly integral to planning and decision-making in healthcare, the levels of expectation, responsibility and accountability increase as well. If patient communities need backing to level up their offering accordingly, other stakeholders can help to support with this as required.
Pharmaceutical companies can be the catalyst
Pharmaceutical companies interact with all key healthcare stakeholders, including patient communities, healthcare professionals (HCPs), regulators, HTA agencies, distributors, investors, and the wider community. Of course, each stakeholder group has its own priorities, but pharmaceutical companies can act as a catalyst and conduit for patient engagement metrics with other stakeholders.
Pharmaceutical companies are in an excellent position to help embed insights and evidence from patient engagement throughout the entire value chain, helping other stakeholders define and measure what matters to patients. This in turn can have a trickle-down effect as stakeholders start to incorporate these patient-focused insights and approaches more systematically. Because pharmaceutical companies often gain vital patient insights at a very early stage of medicine development (e.g., to help inform clinical trial design and endpoints), this information can be invaluable for other stakeholders too.
For regulatory bodies for example, pharmaceutical companies can help to deliver patient-focused evidence, such as co-created PROs, patient preference study data, and patient natural history data, to support regulatory submissions. For rare diseases in particular, gathering and sharing these kinds of insights can be invaluable. The same is true for the HTA process. For HCPs, insights around patient preferences can support shared decision-making.
Pharmaceutical companies can play a central role in working closely with patient communities to help define patient-centric objectives, support patient communities in capability development as needed to have a voice in the healthcare ecosystem, and demonstrate the impact of patient involvement throughout the patient pathway. This provides opportunities for both pharmaceutical companies and patient communities to be central players in healthcare decision-making, while helping to build trust, enhance transparency, and increase accountability.
Conclusions
It is widely accepted that incorporating the patient perspective is important and of value for all stakeholders involved in healthcare. While demonstrating and measuring that value in a tangible way is challenging, it is up to all stakeholders to embrace that challenge, given the potential upsides for patients and indeed the whole of society.
Because pharmaceutical companies interact with all stakeholders, they can help to provide some patient insights and evidence needed for others, and as an ally and partner for patient communities to help their voice to be heard. Pharmaceutical companies can work together with patients to help them co-define patient-centric objectives and metrics to assess the impact of patient engagement activities.
Such a patient-centric approach will also help pharmaceutical companies to demonstrate how their own innovations translate into patient benefits in the real-world – a growing requirement of pharmaceutical companies and growing need for all healthcare ecosystem stakeholders.
Demonstrating the impact of patient engagement is not an easy task. However, pharmaceutical companies have the opportunity and responsibility to play a central role as a catalyst and conduit for all stakeholders, ultimately contributing to better patient outcomes and benefitting all healthcare system stakeholders.
Published in PME Pharmaceutical Market Europe October 2023













