4 Steps to unlocking long-term funding for innovative therapies in the hospital setting

Innovation in the healthcare sector is always welcome to help drive improvements in patient outcomes. However, innovation comes at a price and many stakeholders perceive new innovative therapies, devices or procedures as being a major driver of increasing healthcare costs.
A constant challenge for payers and governments, therefore, is to balance innovation and affordability, and this challenge is in part passed back to the pharmaceutical companies, which increasingly have a larger role in proactively assessing where their new innovations fit within the treatment landscape and how they can be paid for.
Many new (often high-priced) innovations are first used in the hospital setting, where there are two main sources of funding; either via DRG (diagnosis-related groups) or via some form of temporary or permanent add-on funding in addition to DRG.
With DRG-based hospital payment systems, all patients treated by a hospital are classified into a limited number of DRGs. Each DRG is associated with a specific cost. Hospitals then receive a DRG-based case payment; sometimes these payments are subject to a volume agreement. In both cases, hospitals have the potential financial risk of having costs above the payment rate and are rewarded for keeping costs below.
When innovations are associated with increased costs per admission, however, disincentives exist for hospitals to adopt and use them (unless the DRG-based payment system is updated to account for their additional costs, which is uncommon and generally a long, drawn-out process). That is why several countries have developed add-on funding systems to ensure patients can benefit from the latest innovations.
Navigating through a complex environment
Companies need to be able to identify funding opportunities: how innovative hospital drugs are sustainably funded in each market, who are the funding stakeholder types and what are system controls, and identify the long-term funding drivers.
Across countries, different short-term payment systems are used, which provide strong incentives for hospitals to use new innovations because they exempt them from the incentives of DRG-based hospital payment. However, they may also distort clinical decision making and can lead to inefficiencies related to overprovision of these services and to increasing health-care expenditures. Therefore, we are focusing on more sustainable long-term solutions in this paper.
So what are best ways of unlocking long-term funding for high-priced innovative hospital-based therapies?
To find out, Executive Insight conducted qualitative interviews with a range of stakeholders including payers and pharma executives in different markets, to understand the various perspectives. Based on that research, below are four key steps to unlocking long-term funding for innovative products in the hospital setting.
1. Conduct market analyses and understand the impact your new innovation will have on local budgets
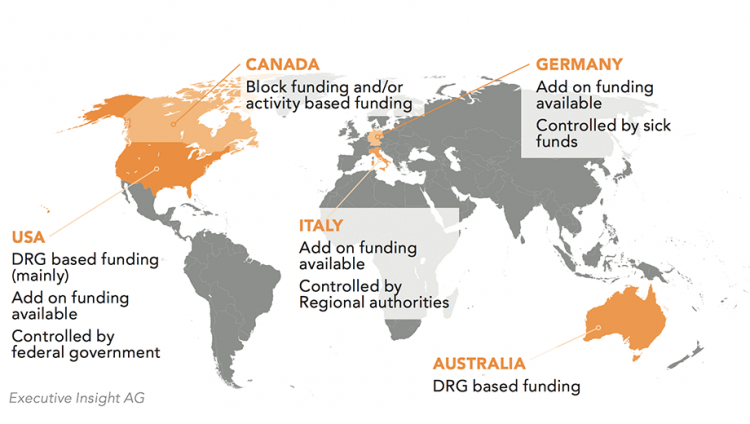
Funding systems vary in each market; you need to know which of your key target markets use only DRG and which employ add-on funding for innovative hospital-based therapies. The map on the following page shows a sample of several key markets and which system they utilise.
You need to understand the impact that your innovation will have on the DRG budget. Where does it fit in and which DRG(s) does it affect? Are the costs offset completely or only partially? What is the overall patient population, the perception of genuine innovation and what is the public profile of the disease?
It is also useful to understand the ability of DRG-based hospital payment systems in each market to respond to new innovations through long-term updating mechanisms. This is determined by the frequency of updates and the time lag between the collection of cost and medical data and using this information for hospital payment.
If you believe your innovation will fall outside the limits of DRG funding, you need to know the specific criteria for securing add-on funding in each market. These differ in each country but there are usually two or three points which need to be adhered to for success. For example, the criteria for New Technology Add-On Payments (NTAP) in the US are: be new (launched within 3 years); offer a significant clinical improvement over existing therapies and; be inadequately paid under the existing Medicare Severity-DRG system.
2. Identify where the budget control lies
Knowing the type of funding opportunities and whether they are likely to cover your new product is half the picture; crucially you need to understand who controls these budgets in each market. Budget holders may carry risks; thus it is important to know those risks so that you can proactively help to manage them accordingly.
For DRG budgets, the individual hospitals generally control the fund allocations. The add-on budget control may lie, for example, with the national health authority (France) or the local insurers (Germany) and the control mechanisms for releasing the money differ significantly.
To secure long term add-on funding there is usually a formal and transparent process, and add-on funding requests have specific deadlines; find out what these are and plan accordingly. Besides the formal process to secure add-on funding, it is also important to understand if any volume agreements or budget caps are placed by the payer on the available add-on funding.
3. Demonstrate value
Whether funding is via DRG or add-on systems, it is imperative for pharmaceutical companies to proactively demonstrate the value of their new innovation.
Particularly for DRG funding, it is important to demonstrate what your product can offer for the patient population in that specific hospital. Examples of hospital-specific endpoints include reduction of length of stay, reduction of re-admissions within 30 days and reduction of in-hospital complications (e.g. hospital acquired infections); these are the endpoints that matter in the hospital setting and will help stakeholders to see how investment can be offset by improved meaningful outcomes.
For add-on funding, the demonstration of value may have to be more wide-ranging. Various activities can be done that directly or indirectly support funding allocation including driving understanding of unmet need, establishing disease advocacy and demonstrating value of use.
4. Segment and target hospitals
Sometimes it may feel as though only barriers exist to new innovations, but that is not the case. All stakeholders want genuinely innovative products and to be a part of something that will make a meaningful difference to patients’ lives. You have to demonstrate the innovation clearly to foster this feeling of excitement and credibility among payers, not just among healthcare professionals.
But it is important to remember that not all hospitals and not all payers are the same: some are more willing to test/introduce innovation than others and some are actively seeking ways to be innovators/leaders in certain fields. It is vital to undertake research to know which ones should be the priority targets for your company.
The segmentation of hospitals (based on innovation, efficiency, reputation, etc.) is therefore absolutely critical to optimise access efforts.
In the longer term, broadening the share of funds dedicated to a specific condition requires a significant investment and effort to raise the public profile of that disease.
Conclusions
Payers always need to tread a fine line between welcoming new innovation and managing cost, particularly in the hospital setting. DRG is a system that overall works well and provides a degree of consistency across countries. However, it is often not enough to cover high-priced innovative new hospital-based therapies; understanding who controls add-on budgets in each market and, crucially, why they decide to allocate funds is vital. Local country affiliates will have a lot of this information, but global teams can support them by helping to analyse the potential impact of a new innovation on local budgets, and by developing compelling packages which demonstrate the value and innovation of the product or service.